Introduction: Treatment-free remission (TFR) is an emerging treatment goal for chronic myeloid leukemia (CML) patients in deep molecular response (DMR). Current evidence shows that 40%-60% of patients relapse while in TFR; and nearly all regain response once tyrosine kinase inhibitors (TKIs) treatment are reinitiated. However a robust predictor of prolonged TFR has not been reported yet. Considering real-life setting, 2 key factors may affect TFR outcome if not properly done: Access to serial molecular monitoring at optimal timepoints and quality laboratory terms as accuracy, sensitivity and rapid results. This motivated the creation of the AST study in our region to guarantee adequate molecular monitoring for TFR in Argentina and characterize new prognostic biomarkers helpful to identify more accurately patients who will be able to sustain TFR. We aimed to assess the proportion of patients with sustained major molecular response (MMR) after TKIs discontinuation and define precise conditions for stopping treatment.
Methods: This prospective, multicentre Argentina Stop Trial (AST) trial is recruiting chronic phase CML patients under TKI treatment for at least ≥ 4 years, in DMR (≥MR4.0) sustained for ≥ 2 years in standardized laboratory, confirmed typical BCR-ABL1 transcripts b3a2 and/or b2a2 and aged > 18 years. Molecular tests are centralized in 2 harmonized laboratories and performed monthly for the first 6 months, every 2 months until the first year, and every 3 months during the second year. If patients lost MMR, TKI was restarted immediately. Molecular relapse Free Survival was estimated by Kaplan-Meier method. Difference between survival variables was evaluated through log-rank test. Multivariate analysis was performed through Cox proportional hazards model. The cutoffs of the numerical variables were considered according to the log-rank test.
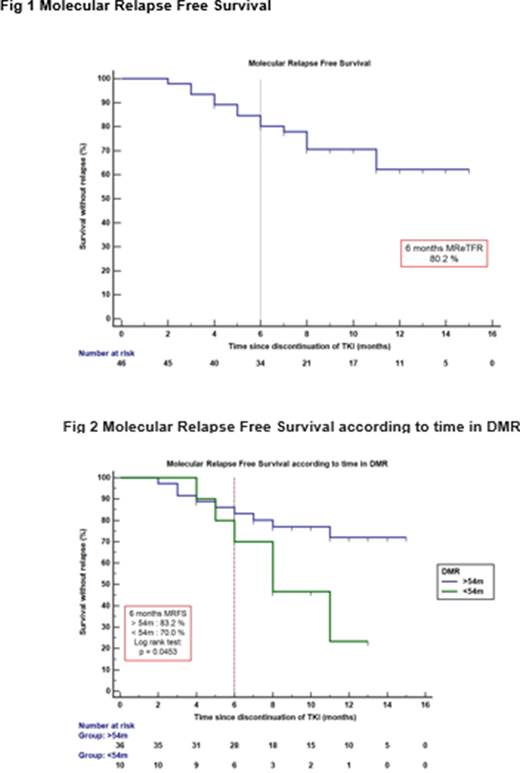
Results: Between February 2019 and July 2020, we evaluated 50 CML patients of whom 46 were enrolled from 7 centers in Argentina and 4 were screening failures. Recruitment was interrupted due to COVID-19 pandemic. Patient median age was 57.5 years (range 24-85). Before discontinuation, TKI treatment was as follows: Imatinib 37/46 (80%), Nilotinib 5/46 (11%) and Dasatinib 4/46 (9%), 2G-TKI as 1st line, 11% of the patients received non-branded treatment. Sokal risk score showed to be low in 22 patients (48%), intermediate in 14 (30%) and high in 10 (22%). Median follow-up was 10 months (range 4-17) and the estimated molecular relapse-free survival was 80.2% (95%CI 69-93) at 6 months Fig 1. Longer DMR durations before discontinuation were associated with increased probability of maintaining response at 6 and 12 months: 83.2% for patients who had >54 months in DMR vs 70% with <54 months and 72% vs 23.3% respectively (p=0.0453) Fig 2. Cox multivariate analysis was performed including different variables as age at diagnosis, time in DMR, time in TKI previous to discontinuation and Sokal risk. The only significant variable associated to improved prognosis was time in DMR (HR 2.8 95%CI 1.002-8.07 p=0.0495). Our cohort had a long time on TKI treatment previous to discontinuation, median 10.5 years (4.16-17.5) probably considering it a favorable factor for the high TFR rates described at 6 months. Among the 46 patients included, 15 (33%) lost MMR, all restarted treatment with the same TKI used before discontinuation, 12/15 (80%) regained MMR with a median time of 3 months (range1-8) and 9/15(60%) obtained MR 4.0 with a median time of 3 months (range1-5).
Conclusion: This is the first multicenter study of TKI discontinuation in CML patients in Argentina showing that TKI can be safely discontinued in those who achieve and maintain a DMR before discontinuation. We observed high rates of molecular relapse free survival, although longer follow-up is needed. We must continue with this approach for patients participating in TFR trials or TFR programs in order to decrease the risk of relapse and make this goal a fact in our region. This discontinuation study will allow in a near future significant saving of economic resources and might improve patients quality of life specially in those who are currently experiencing treatment adverse events.
Pavlovsky:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Speakers Bureau; Pfizer: Speakers Bureau; Pint Pharma: Speakers Bureau. Varela:Novartis: Consultancy, Speakers Bureau. Pavlovsky:Janssen: Membership on an entity's Board of Directors or advisory committees, Other: travel grants, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Speakers Bureau; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Varifarma: Speakers Bureau. Moiraghi:BMS: Speakers Bureau; Novartis: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal